Are you a pharmacist who dispenses drugs for animals?
Then you might already know that differences between species can be dramatic—and can have major implications for prescribing and dispensing drugs.
To further complicate veterinary prescribing, many human drugs aren’t labeled for animal use but can be prescribed by veterinarians for certain species and indications anyway. In addition, a veterinary drug may only be FDA-approved for use in one species—but can be prescribed for multiple.
This is permitted by the Animal Medicinal Drug Use Clarification Act of 1994 (AMDUCA), which allows veterinarians to prescribe FDA-approved animal and human drugs in an extra-label manner under certain conditions.
Given these factors, how can pharmacists determine which medications are appropriate to dispense to animals?
How to know what drugs and dosages you can dispense to animals
All the drug information in Plumb’s Veterinary Drugs® is specific to animals, making it the trusted reference pharmacists need to safely dispense drugs for veterinary patients.
And it’s easy to find the information you need. Using the Plumb’s™ web or mobile app, simply type a drug or trade name into the search for quick results.

In each drug monograph, you’ll find all the animal-specific information you need, like dosages, drug formulations, uses and indications, contraindications, and adverse effects.
Whether you’re dispensing for a dog or a cat (or even a lizard), you’ll find species-specific dosing information in the Dosages section. There, you’ll encounter one of three scenarios.
Dosage details you should know about
1. Your patient’s species is not listed in the dosages section.
If there’s no dosing information listed for your patient’s species, it means no established and/or safe dosage has been determined for that drug in this species.
For example, look at the dosages section of the acetaminophen monograph. There’s dosing information for dogs, horses, and rabbits/small mammals but not for cats or ferrets—because acetaminophen shouldn’t be used—at any dosage—in these species.
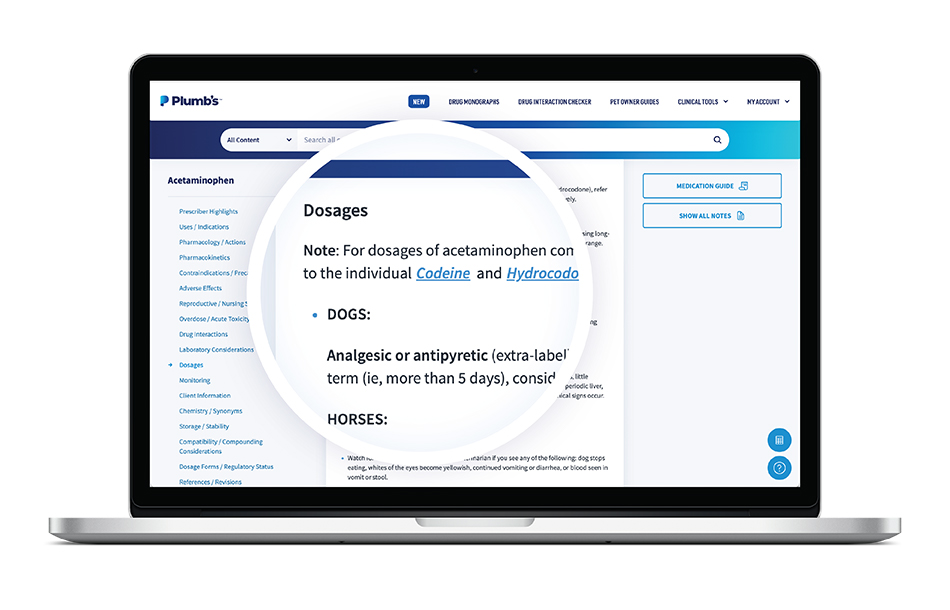
2. Your patient’s species is listed, and “label dosage” appears beside the indication.
If you see a label dosage, you know there’s an FDA-approved veterinary formulation of the drug, and the dosage that’s listed is intended for the specific indication and species defined on the drug label.
For example, enrofloxacin is available as a trade-name product (Baytril®) in veterinary medicine and the label dosage is specific for the treatment of susceptible infections in dogs.

3. Your patient’s species is listed and “extra-label” appears beside the indication.
When you see “extra-label”, you know that while the drug itself is approved for humans and/or animals, it’s being used in a way that isn’t described on an FDA-approved veterinary label.
The most common types of extra-label dosages include:
- The drug has been approved for use in humans but is not specifically approved for animal use—so there is no veterinary label.
- An FDA-approved veterinary drug is being used but not in the way that is described on the veterinary label. For example, the drug is being used:
- In a species not listed on the label
- For indications not listed on the label
- Via a different route of administration or at a different dosage or frequency than what is stated on the label
For example, when enrofloxacin is used to treat susceptible infections in dogs, it’s being used as indicated on the label. But if it’s being used in a species not listed on the veterinary label or for a different indication—like pyelonephritis—its use is extra-label.

For this reason, you’ll often find both label and extra-label dosages listed when drugs are used for a variety of species and indications.
Where does extra-label drug information in Plumb’s™ come from?
The decision to publish extra-label drug information is made by our team of veterinary and pharmacy experts after examining the peer-reviewed literature. Once substantial clinical evidence exists for the safe use of a drug in animals, the information is added to the drug monograph.
And all the content in Plumb’s Veterinary Drugs® is continually updated to ensure it remains accurate.
How do I know if drugs can be safely used together?
A wide variety of drugs are used in animal patients, and there are vast drug-related differences between species. So how can you avoid potential drug interactions when an animal is on multiple drugs?
Human-specific drug interaction tools typically don’t list veterinary drugs and indications or reference species-specific data—leaving a dangerous information gap.
But Plumb’s™ has you covered with the first-of-its-kind veterinary drug interaction checker. This easy-to-use tool is animal-specific and can evaluate drug-to-drug interactions between all the drugs your patient is on.

The drug interaction checker will automatically populate a list of drug-to-drug interactions and specify a risk level for each combination, helping you safeguard your patients against drug interactions.
With Plumb’s™ by your side, you have all the information you need to safely dispense medications for animals.
Don’t have Plumb’s™ yet? It only takes a few minutes to subscribe.
For more information on prescribing for pets versus people, check out our pharmacist’s guide to filling veterinary prescriptions.
Have questions? Just contact us—we’re always happy to help.


